Abstract
Background OVIDIO (ObserVational study in patients with aDvanced systemic mastocytosis treated in Italy with midOstaurin) is a multicenter, non-interventional study describing a cohort of adult patients (pts) with advanced systemic mastocytosis (AdvSM) treated with and without midostaurin in clinical practice, to fill the knowledge gap between the clinical experience from registration trials and routine pt management in Italy.
Methods Adult pts with AdvSM were enrolled into one of two cohorts: 1) pts with AdvSM who were treated with midostaurin (according to local label) from Jan 2016 (MT); 2) pts with AdvSM who were diagnosed between Jan 2010‒Dec 2015 and did not receive midostaurin (MNT). The primary objective of this study is to describe the profile and disease burden of the two cohorts. Secondary objectives include the characterization of clinical management in both cohorts and, for the MT cohort, the evaluation of the efficacy (according to IWG-MRT-ECNM criteria) and safety of midostaurin in AdvSM. Here we report an interim analysis of pts in the MT cohort who completed 12 months of follow-up or discontinued treatment within 12 months.
Results At data cut-off (Jan 31, 2022), the MT cohort included 66 pts, of which 54 were evaluable; reasons for exclusion were inclusion criteria not met (n=1) and <12 months of treatment (n=11). At 12 months, 42 (77.8%) pts were ongoing in the study and 12 (12.2%) had discontinued; reasons for discontinuation were death (n=10), loss to follow-up (n=1) and physician decision (n=1). Median age at diagnosis was 66.0 (range 31-84) years (Table). Evolution to AdvSM from non-AdvSM was reported in 16 (29.6%) pts. According to WHO 2016 criteria, 20 (37.0%) had SM-AHN, 32 (59.3%) had ASM and 2 (3.7%) had MCL. Among pts with SM-ANH, most pts had CMML (n=6; 30%) as an associated neoplasm. KITD816V mutational status was assessed in 44 (81.5%) and 12 (22.2%) pts at diagnosis and baseline, respectively. Of pts with KITD816V mutational status assessed, allele burden was evaluated in 14 (31.8%) and 6 (50.0%) of pts at diagnosis and baseline, respectively. Mean (SD) allele burden at diagnosis and baseline were 13.5% (12.8) and 9.5% (11.6), respectively.
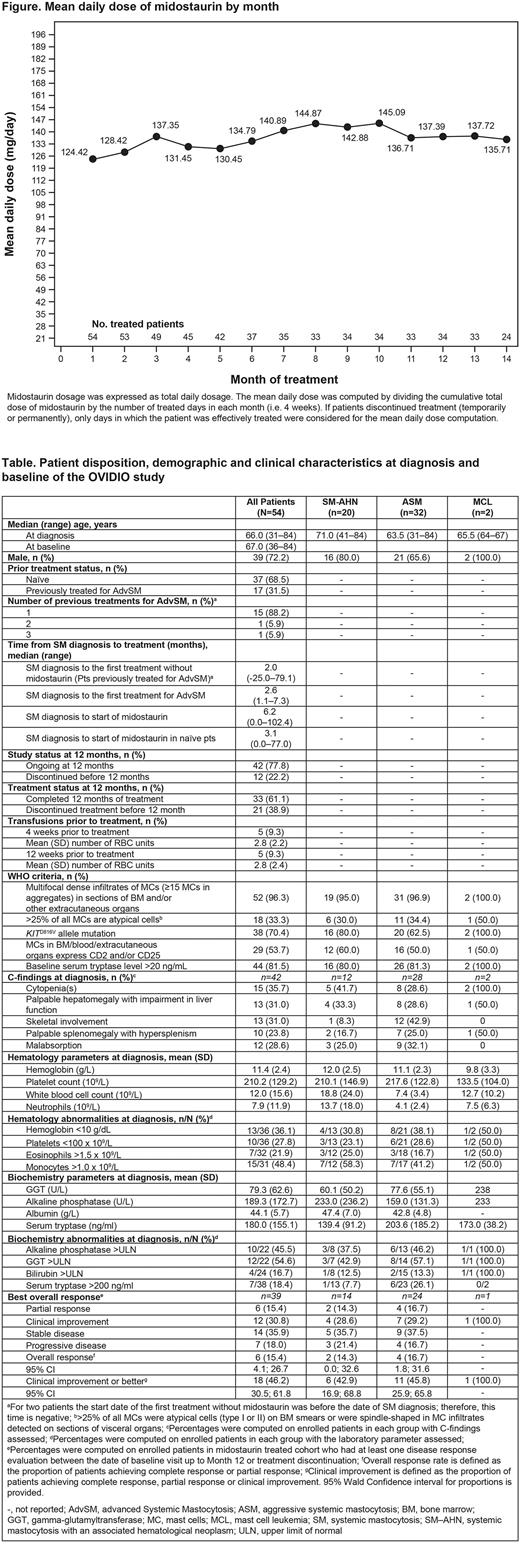
Median time from diagnosis to midostaurin start was 6.2 (range 0.0-102.4) months. In total, 17 pts had received prior treatment for AdvSM, including cladribine, interferon α-2a, hydroxycarbamide and imatinib. The initial mean (SD) daily dose of midostaurin was 114.4 (60.8) mg (16 [29.6%] pts started treatment at 200 mg daily) and the mean (SD) dose during the 12 months of follow-up was 132.4 (47.2) mg (Figure). Thirty-three (61.1%) pts completed 12 months of treatment. Temporary treatment interruption occurred in 21 (38.9%) pts; the most frequent reason was an adverse event (AE; n=21, 87.5%).
Disease response was assessed in 39 pts; 18 (46.2%) pts had a clinical improvement or better (Table). Post-baseline serum tryptase was assessed in 14 pts with a best median reduction from baseline during the 12 months of follow-up of 43.2%.
Overall, 44 (81.5%) pts had an AE and 11 (20.4%) had a serious AE (SAE). AEs and SAEs related to midostaurin occurred in 35 (64.8%) and 7 (13.0%) pts, respectively. The most frequently reported treatment-related AEs were gastrointestinal disorders (44.4%). Five (23.8%) pts permanently discontinued treatment due to AEs and 3 (5.6%) pts had fatal SAEs.
Conclusions This interim analysis of the MT cohort demonstrated a heterogenous diagnostic and follow-up approach across Italian sites. Results are overall consistent with previous reports, but pts received a mean daily dose lower than expected. Most pts continued treatment over the 12-month follow-up period and few pts discontinued midostaurin due to AEs.
This study was sponsored by Novartis Farma SpA.
Disclosures
Papayannidis:Abbvie: Honoraria; Amgen: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Blueprint: Honoraria; Incyte: Honoraria; GlaxoSmithKline: Honoraria; Bristol Myers Squibb: Honoraria. Mannelli:Blueprint: Speakers Bureau; Novartis: Speakers Bureau. Elena:Blueprint: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Criscuolo:Novartis: Speakers Bureau. Giona:Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding. Pane:Novartis: Research Funding, Speakers Bureau. Bini:Novartis Farma SpA: Current Employment. Valsecchi:Novartis: Current Employment. Grifoni:Novartis: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal